By Edmar Ramos de Oliveira Filho
As the transition away from fossil fuels occurs, efforts to move from a linear to a circular economy are necessary. Biotechnology can provide sustainable and environmentally friendly solutions. This includes new processes for biomanufacturing innovative products from renewable sources, thereby avoiding the use of petroleum-based feedstocks and helping to reduce greenhouse gas emissions. Agricultural byproducts are one of the most promising renewable carbon-rich feedstocks to be upcycled since they are cheap and abundant.
What are the Main Agricultural Byproducts?
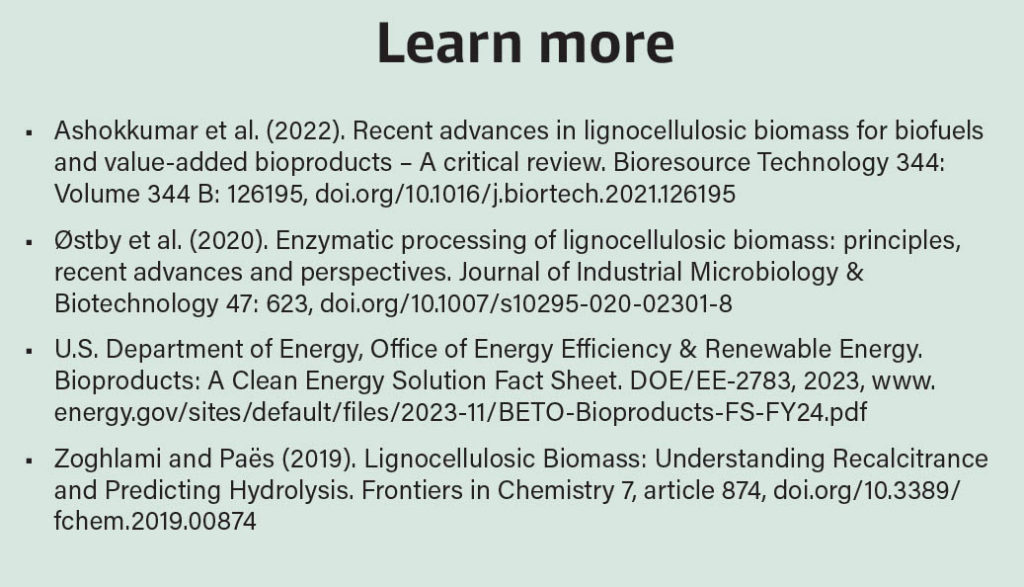
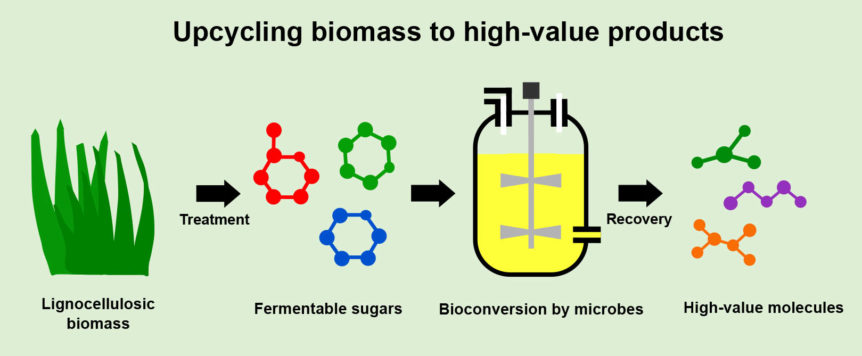
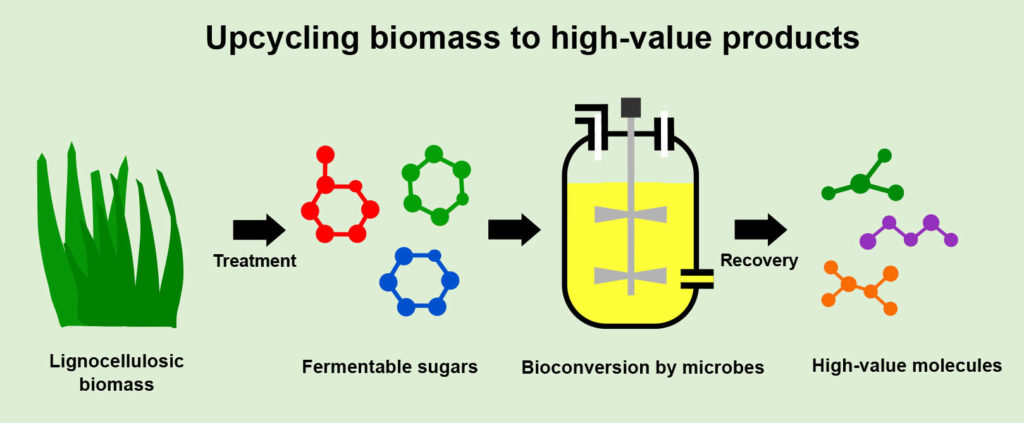
Agricultural byproducts (or residues) like corn stover, sugarcane bagasse, citrus peel and wheat straw are usually classified as waste biomass. These constitute lignocellulosic biomass, the world’s most abundant renewable feedstock. Such residues are often disposed of or burned to generate heat or electricity in the production plant. Lignocellulose is mainly composed of cellulose, hemicellulose (both sugar polymers) and lignin (an aromatic polymer). When submitted to a complex treatment (involving high temperatures and enzymes), useful molecules such as glucose, xylose, arabinose, cellulose, lignin and aromatic compounds can be obtained from such lignocellulosic residues. Obtaining high yields of these is still a problem, however, and much effort is being put into solving it, aiming for the development of suitable treatment options.
How Can These Residues Be Converted?
Some microbes (bacteria and yeast) can grow and produce valuable chemicals using the molecules derived from agricultural residues. However, in most cases, their efficiency is not yet sufficient for large-scale industrial production. Researchers and industry experts are actively seeking new microbial strains that can utilize these residues more efficiently.
Another approach involves genetically modifying well-established industrial workhorses, such as the bacterium Escherichia coli or baker’s yeast. These genetic engineering strategies can, for example, empower the workhorse microbes to grow using a specific molecule, speed up the microbe’s growth rate and increase the quantity of product generated from a given amount of starting material. Furthermore, exploring new microbial species expands the pool of genes that can be introduced into classical industrial strains, making them more suitable for large-scale production. By combining all these strategies, bulk and specialty natural or new-to-nature products can be produced. This includes biofuels (e.g., bioethanol and biodiesel), bioplastics (e.g., polyhydroxyalkanoates), biosurfactants (e.g., rhamnolipids), organic acids or precursors that can then be chemically converted to a range of profitable molecules.
What is Still Necessary?
A major obstacle to upcycling lignocellulose is its resistance (recalcitrance) to most of the treatments that can be used to break it down into compounds that bacteria or yeast can grow on. To improve the accessibility of lignocellulose to degrading enzymes, a pretreatment is often necessary, such as acid treatment, ammonia fiber expansion or steam explosion. The enzymatic treatment will then break the polymers into smaller molecules that the microbes can use. It is important to stress that both the pretreatment and the composition of the enzyme cocktail must be adjusted based on the characteristics of the feedstock. A viable method depends on economically feasible pretreatment and enzymatic breakdown processes.
Therefore, it is necessary to continue developing new affordable technologies to increase the yields of fermentable compounds (e.g., glucose, xylose and arabinose) obtained from crop waste. Establishing an ideal treatment is not easy. Some of the chemicals and enzymes used typically co-generate toxic compounds (non-fermentable molecules) that can kill the microbes or inhibit their growth. To overcome this toxicity, robust microbes that grow in the presence of such inhibitors can be identified and genetically manipulated to produce target value-added products.
In addition, it is crucial to estimate how realistic a biotechnology project aiming for the industrial production of a valuable molecule actually is. Simple calculations can be used for this. For example, consider the availability of a crop waste (e.g., tons per year) and how much of it can be converted into a desired product using the current technology (grams of product obtained per gram of substrate). With such simple but reliable estimates, scalability and outcomes can be assessed, and areas for improvement can be identified.
Edmar Ramos de Oliveira Filho is a postdoctoral associate with the University of Florida Institute of Food and Agricultural Sciences in Gainesville.